|
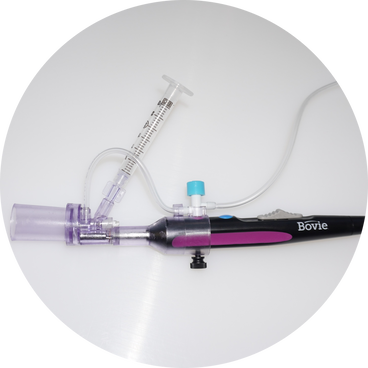
Our Vision AerMedX is also working on a series of future devices that can apply therapeutic biologics and pharmaceuticals directly onto wounds, burns and damaged tissues using our proprietary processes. This will include systems for depositing antibiotics onto surgical sites and biomolecules to enhance wound healing. |
|
Our Technology AerMedX utilises a revolutionary coating technique called BioDep. The BioDep process was developed by our associated company TheraDep. To find out more about the BioDep process please visit TheraDep's website at the link below. |
Disclaimer:
Note: AerMedX deposition accessory is an investigational device and can be used only in the setting of an FDA-approved clinical trial. The purpose of this website is only to obtain investigators and not to make the device generally available.
Caution: Investigational device, limited by United States Law to investigational use only.
Note: AerMedX deposition accessory is an investigational device and can be used only in the setting of an FDA-approved clinical trial. The purpose of this website is only to obtain investigators and not to make the device generally available.
Caution: Investigational device, limited by United States Law to investigational use only.
Read more:
- Wound healing using plasma modified collagen - Clinical Plasma Medicine
- Evaluation of the J-Plasma Electrosurgical Device Combined with Nebulized Collagen for Burn Healing in Rodents - Plasma Medicine
- A comparison of two cold atmospheric helium plasma devices which utilise the same RF power generator - Clinical Plasma Medicine